The scientific teams of Oncodesign, Cyclopharma, and their collaborators within the IMAkinib® program published recently their results during international meetings. These poster publications presented the synthesis of a radiotracer targeting the EGFR kinase, and the preclinical development of the molecule:
Application of the Nanocyclix® technology to identify clinically relevant PET
In vitro and in vivo evaluation of two enantiomers of Nanocyclix® EGFR targeted PET radiotracer
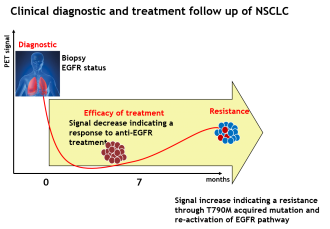
The first radiotracer developed within IMAkinib® program has been succesfully evaluated in phase 0/1 clinical trial (first in-man phase 0/I clinical trial NCT02847377). The objective of this clinical phase was to determine the molecule biodistribution in patients, and confirm its specific binding in tumors harboring EGFR mutation in comparison with EGFR non-mutated tumors. This clinical study highlights the expertise of our partners as it is the first phase 0 performed at the center of early investigation within the Georges François Leclerc Centre and the first production of a radiotracer dedicated to clinical research done by Cyclopharma in the radiochemistry laboratory of Pharm’image.
These concrete achievements represent the results from the last 10 years research investments carried out on pharmaco-imaging development within the Pharm’image biocluster with all actors, from chemistry to clinic, involved in new radiopharmaceutical drug development within Pharm’image.
A complete presentation of this radiotracer development, including the clinical trial design, is also available on Oncodesign website: