Overview
- To complete Oncodesign preclinical MRI capabilities, the Pharm’image preclinical imaging platform was created in 2011 in the nuclear medicine department of the CGFL (the region’s comprehensive anti-cancer center).
- This infrastructure which is dedicated to preclinical imaging, is accredited by the French authorities for both the handling of radioactivity (ASN) and animal housing (DDPP).
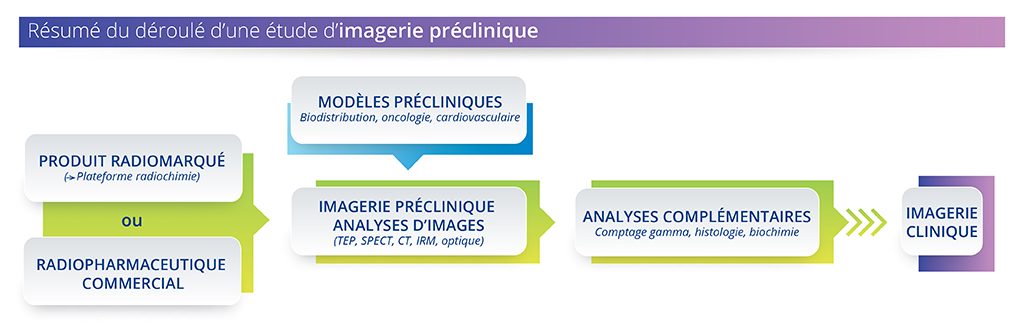
- Preclinical and clinical imaging share the same imaging modalities and biomarkers. Preclinical imaging is therefore a translational tool that can reduce the costs and timelines of the drug development process, by narrowing the gap between basic science and clinical proof of concept.
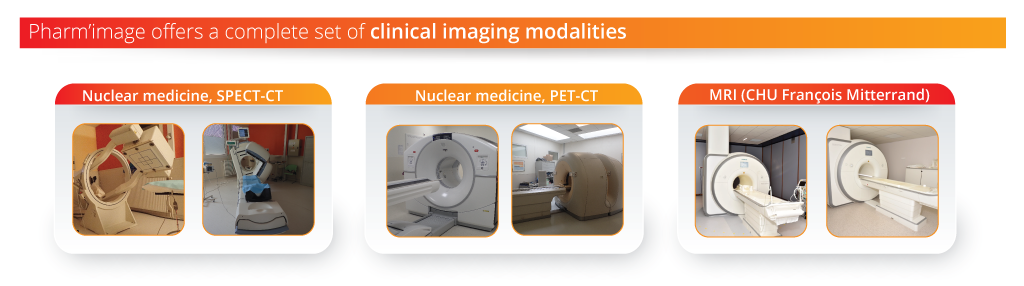
Equipment

Applications
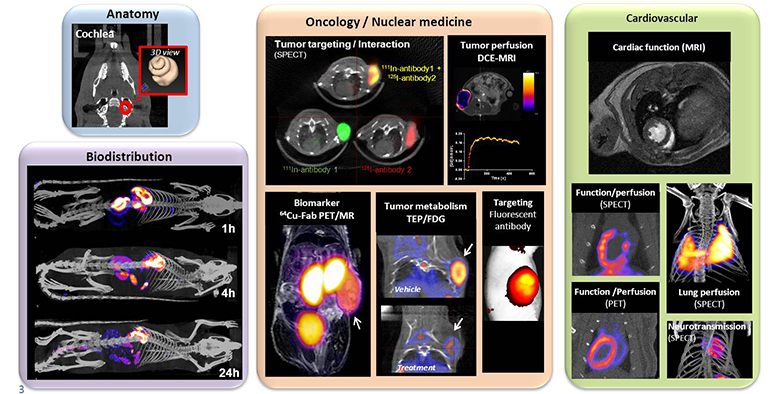
Numerous imaging endpoints (morphological, functional and molecular) were developed in different areas: Pharmacology, Oncology, Cardiology, and Nuclear Medicine.